Market Overview:
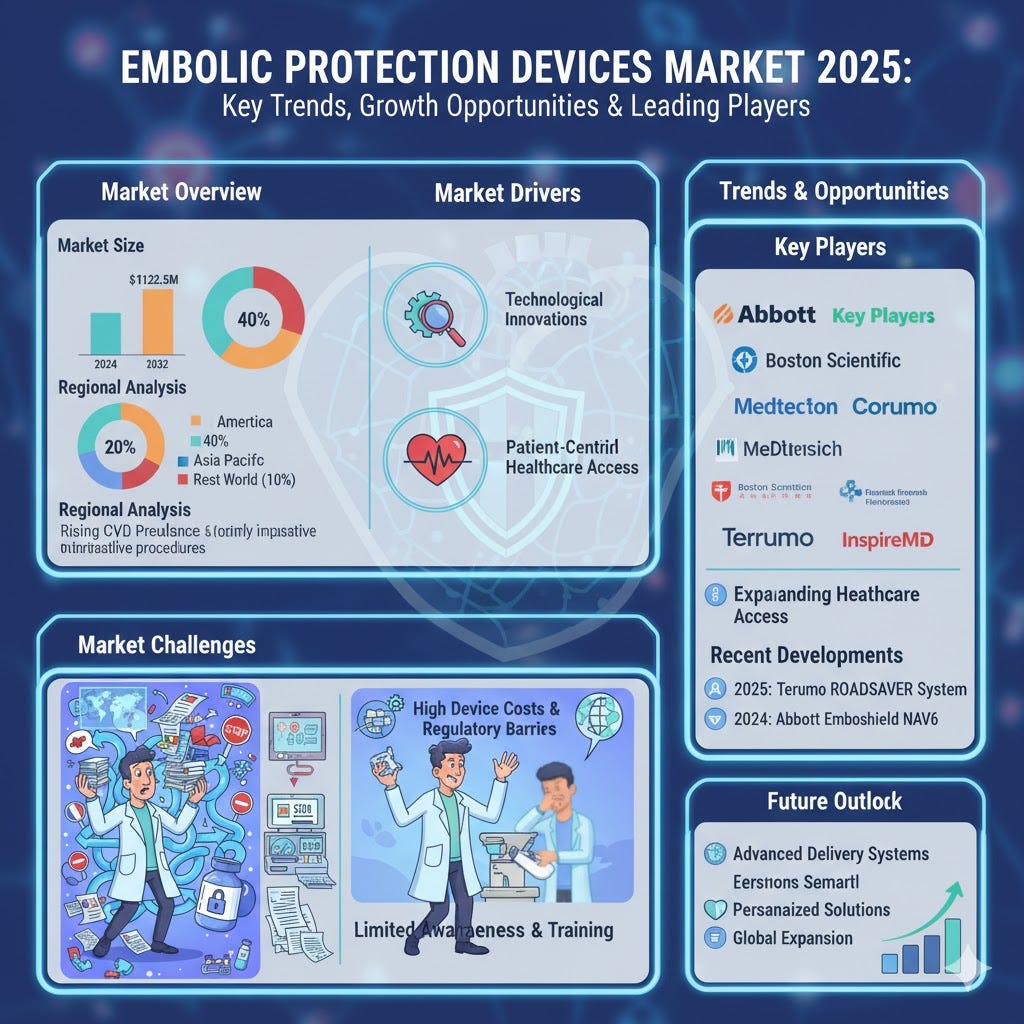
The Embolic Protection Devices Market size was valued at USD 584.5 million in 2024 and is projected to reach USD 1,122.6 million by 2032, reflecting strong growth. As per Credence Research, innovations in device design are enhancing procedural safety and efficacy during carotid artery stenting, coronary interventions, and peripheral procedures. Technological advancements, including smaller and more flexible devices, improve patient outcomes and increase adoption. The rising prevalence of cardiovascular diseases, coupled with the shift toward outpatient surgeries and preventive healthcare, accelerates demand. North America leads the market, followed by Europe, with Asia Pacific showing the highest growth potential.
Source: https://www.credenceresearch.com/report/embolic-protection-devices-market
Market Drivers:
Technological Innovations Enhancing Safety and Precision
The Embolic Protection Devices Market benefits from continuous innovation, introducing flexible, efficient, and compact devices that improve procedural precision and patient safety. It supports minimally invasive interventions, enhancing outcomes in carotid and coronary procedures. Device advancements, such as advanced filter systems, allow optimal debris capture without compromising blood flow. Hospitals and clinics increasingly adopt these innovations, expanding procedural success and patient satisfaction.
Rising Cardiovascular Diseases and Outpatient Procedures
Increasing prevalence of cardiovascular diseases globally drives the need for embolic protection devices. It supports preventive and therapeutic interventions for conditions like atherosclerosis, stroke, and coronary artery disease. Growing adoption of outpatient and minimally invasive procedures further boosts demand. Healthcare providers prioritize devices that reduce recovery time, lower risks, and improve efficiency, reinforcing market expansion. Emerging markets also benefit from rising awareness and improved healthcare infrastructure.
Market Trends and Opportunities:
Shift Toward Patient-Centric and Personalized Devices
The Embolic Protection Devices Market witnesses growing focus on patient-specific solutions. It supports devices tailored to individual vascular conditions, improving safety and efficacy. Advanced diagnostics enable better device selection, ensuring optimal protection. Manufacturers are designing filters and stents in multiple sizes for varied patient anatomies. The trend toward personalized interventions opens opportunities for innovation and market differentiation.
Expanding Healthcare Access and Regional Growth Potential
Growing access to advanced healthcare technologies in Asia Pacific and emerging markets drives adoption. It supports the increasing number of cardiovascular procedures, including carotid and coronary interventions. Rising investments in medical infrastructure and patient awareness fuel market expansion. Collaborations between device manufacturers and hospitals enhance product reach and adoption, creating growth potential across developing regions.
Market Challenges:
High Device Costs and Regulatory Barriers
The Embolic Protection Devices Market faces challenges due to high equipment costs and complex regulatory approvals. It limits adoption in price-sensitive markets. Strict compliance requirements and lengthy approval timelines slow product launches. Healthcare providers may hesitate to invest in expensive devices despite clinical benefits, restraining growth.
Limited Awareness and Procedural Training
Adoption is hindered by insufficient awareness and training among healthcare professionals. It requires skilled operators to maximize device efficacy. Limited training programs and procedural expertise can affect outcomes and slow market penetration. Emerging regions may struggle to implement advanced technologies without adequate educational initiatives.
Regional Analysis:
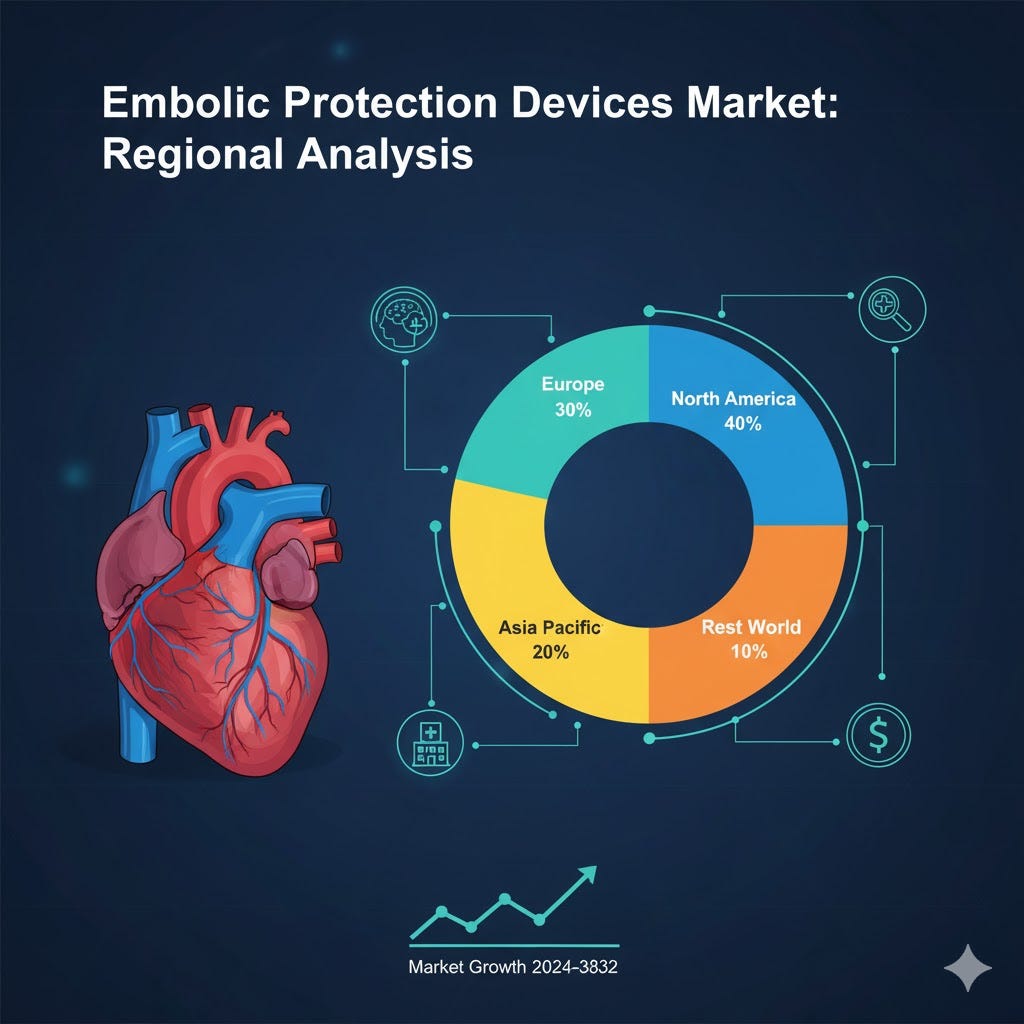
North America: 40% market share; strong healthcare infrastructure, high adoption of minimally invasive procedures.
Europe: 30% market share; aging population, increasing awareness of cardiovascular health.
Asia Pacific: 20% market share; highest growth due to expanding healthcare access and rising CVD prevalence.
Rest of the World: 10% market share; growing awareness and gradual adoption of advanced devices.
Key Players:
Abbott Laboratories
Boston Scientific Corporation
Terumo Corporation
Medtronic Plc
Cordis Corporation
InspireMD Inc.
Go-To Market Strategy:
The [Embolic Protection Devices Market] leverages partnerships with hospitals, cardiology centers, and distributors to expand reach. It focuses on clinician training, product demonstrations, and evidence-based marketing campaigns. Device manufacturers engage in collaborations with research institutions to showcase efficacy and safety. Strategic pricing, patient awareness programs, and regional adaptation further strengthen market penetration. Adoption of digital marketing, workshops, and conferences enhances visibility, while regulatory compliance ensures smooth entry in new markets. Targeting high-volume cardiovascular procedure centers and emerging regions supports sustained growth and broad acceptance of embolic protection solutions.
Recent Development:
2025: Terumo Interventional Systems launched ROADSAVER Carotid Stent System with dual-layer micromesh design.
2024: Abbott introduced Emboshield NAV6 with innovative BareWire technology for improved debris capture.
2023: Boston Scientific expanded Sentinel Cerebral Protection System with multiple filter sizes for tailored interventions.
Future Outlook:
The Embolic Protection Devices Market is expected to expand steadily with rising cardiovascular disease prevalence and increasing minimally invasive procedures. It will benefit from innovations in device design, personalized solutions, and enhanced patient safety. Growing adoption in emerging markets and expanding healthcare infrastructure will accelerate demand. Focus on outpatient procedures and preventive healthcare supports wider deployment. Collaborations between device manufacturers and hospitals will enhance market penetration. Improved clinician training and awareness initiatives will facilitate effective usage. Regional growth in Asia Pacific and Latin America offers significant opportunities. Overall, market expansion is likely to continue robustly.
Source: https://www.credenceresearch.com/report/embolic-protection-devices-market