The Acceleration of Advanced Medicine: North America Cell and Gene Therapy CDMO Market Poised for Explosive Growth

The landscape of modern medicine is undergoing a profound transformation, driven by the emergence of Cell and Gene Therapy (CGT). These advanced therapeutic modalities, which include curative treatments for previously intractable diseases, are redefining healthcare — and creating an unprecedented demand for specialized manufacturing expertise. At the heart of this revolution is the North America Cell and Gene Therapy CDMO Market, a sector experiencing accelerated growth and strategic evolution.
According to market analysis data, the North American market for Contract Development and Manufacturing Organizations (CDMOs) specializing in CGT is on a trajectory of monumental expansion. According to Credence Research valued at a substantial USD 2,813.27 million in 2024, this critical biomanufacturing segment is anticipated to surge to an impressive USD 11,167.45 million by 2032. This explosive growth is underpinned by a robust Compound Annual Growth Rate (CAGR) of 17.54% during the forecast period of 2025–2032, highlighting the region’s dominance in advanced therapies and biotechnology.
The foundational shift toward these cutting-edge treatments necessitates complex, high-quality, and scalable manufacturing solutions. For pharmaceutical and biotech companies, partnering with a specialized CGT CDMO is no longer a luxury but a strategic necessity. This outsourcing trend allows innovators to focus on their core competencies discovery and clinical development — while entrusting the intricate challenges of viral vector manufacturing and cGMP manufacturing to expert partners.
Source: https://www.credenceresearch.com/report/north-america-cell-and-gene-therapy-cdmo-market
Key Drivers Fueling North America’s CDMO Momentum
The remarkable forecast for the North America Cell and Gene Therapy CDMO Market is propelled by a confluence of powerful market drivers, technological advancements, and a supportive regulatory climate.
1. Growing Demand for Advanced Therapies Across the Healthcare Ecosystem
The primary driver is the rising success and expanding pipeline of advanced therapies. These therapies, particularly in the fields of oncology (such as CAR-T and CAR-NK cell therapies) and genetic disorders, are demonstrating curative potential, which, in turn, fuels the need for commercial and clinical supply. The increase in global clinical trial activity, especially across North America, directly translates into higher demand for CDMO services, covering everything from process development to clinical-stage manufacturing.
A case in point is the proactive expansion by industry leaders. For instance, in August 2022, Thermo Fisher Scientific significantly expanded its cell and gene therapy CDMO capabilities by opening a massive 300,000-square-foot facility in Plainville, Massachusetts, specifically designed for large-scale viral vector production to support the booming demand in the oncology space. This strategic expansion is indicative of the industry-wide commitment to scaling capacity.
2. Expanding Regulatory Support and Accelerated Approval Pathways
The supportive regulatory landscape in the United States, primarily driven by the U.S. FDA, is a major catalyst for market growth. The agency continues to provide expedited approval pathways and designations like Breakthrough Therapy and Orphan Drug Status. This favorable environment encourages faster commercialization and de-risks the investment for developers, thereby boosting demand for CDMO partners who can navigate stringent cGMP compliance requirements and accelerate time-to-market. Clear regulatory oversight also builds investor confidence and strengthens the foundation for therapeutic development across the region.
3. Increasing Investments and Capital Inflows into Biotech Development
North America benefits from a dense ecosystem of venture capital and private equity dedicated to life sciences. Large-scale biotechnology investment supports both biotech firms and their CDMO partners, enabling the construction of new facilities and the adoption of cutting-edge manufacturing platforms. High-value funding rounds provide the critical financial resources needed to advance complex cell and gene therapy programs.
A notable example of this capital inflow is the ElevateBio transaction in May 2023. The company closed a significant $401 million Series D funding round, with proceeds earmarked to advance its cell and gene therapy CDMO capabilities. This included the expansion of its BaseCamp® end-to-end cGMP manufacturing and process-development business in Massachusetts, a direct testament to the market’s investment potential.
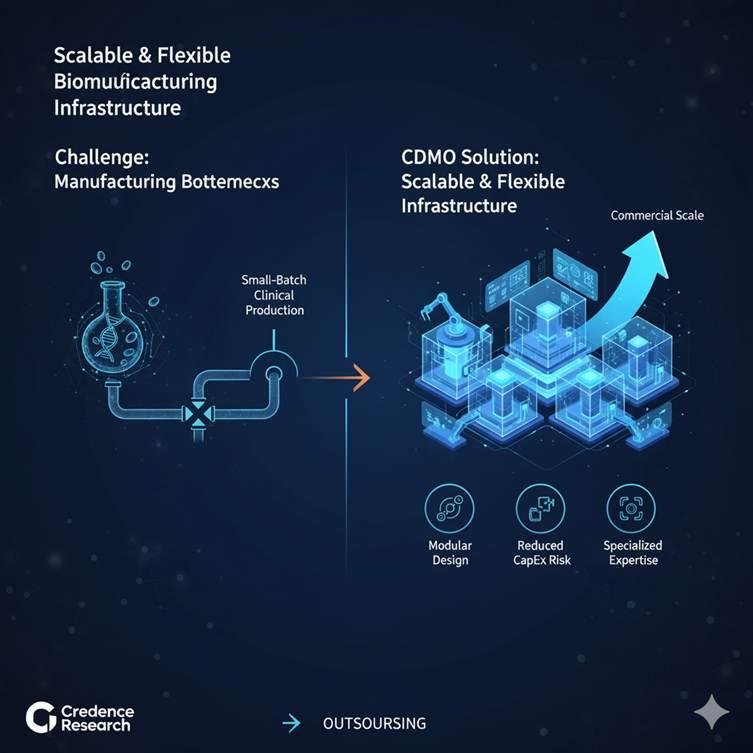
4. Rising Need for Scalable and Flexible Manufacturing Infrastructure
The inherent complexity of cell and gene therapy products creates significant manufacturing bottlenecks, particularly when transitioning from small-batch clinical production to commercial scale. This has resulted in an urgent requirement for highly scalable and flexible biomanufacturing infrastructure. CDMOs are uniquely positioned to offer modular, multi-product facilities that meet the diverse needs of sponsors, reduce capital expenditure risks, and provide specialized technical expertise that is often lacking in-house. This necessity continues to drive outsourcing as sponsors seek compliant, efficient, and adaptable solutions.
Revolutionary Market Trends Shaping the Future
Beyond the core drivers, several key trends are defining the strategic direction and competitive landscape of the North America Cell and Gene Therapy CDMO Market.
Integration of Advanced Automation and Digital Manufacturing Solutions
To address the challenges of variability, human error, and cost, the market is rapidly integrating advanced automation and digital manufacturing solutions. AI-driven platforms and closed-system manufacturing are becoming standard practice to improve process consistency, enhance efficiency, and maintain regulatory compliance. CDMOs are applying advanced monitoring systems and data analytics to optimize cell quality and viability.
An illustration of this technological shift is the April 2024 collaboration between Bristol Myers Squibb (BMS) and Cellares. Their $380 million capacity reservation and supply agreement centers on deploying Cellares’s fully automated Cell Shuttle platform across smart factories. This move demonstrates a clear industry commitment to leveraging automation for commercial-scale manufacturing of complex CAR-T cell therapies.
Growing Focus on Allogeneic Therapies and Platform Development
A major trend is the strategic pivot toward allogeneic therapies. Unlike patient-specific autologous therapies, allogeneic “off-the-shelf” treatments can be produced in large batches from a single source, dramatically improving scalability, access, and potentially reducing per-dose costs. CDMOs are heavily investing in platform-based models to streamline the development and manufacturing of these universal donor-derived products, including strengthening expertise in cryopreservation and cold-chain logistics.
Expansion of Strategic Collaborations Across Biopharma and CDMOs
Strategic collaborations are consolidating expertise and accelerating time-to-market. Partnerships between large pharmaceutical companies and specialized CDMOs are becoming a fundamental business model, allowing large entities to quickly access specialized manufacturing expertise and innovative platforms without the years of required internal investment. The aforementioned agreements with Thermo Fisher, ElevateBio, and the landmark deal between BMS and Cellares underscore this pervasive trend of strategic alliances that enhance value chains and drive innovation in the biotech sector.
Increasing Emphasis on Patient-Centric Therapy Development Models
The market is increasingly adapting to a patient-centric model — a core demand of personalized medicine. This shift focuses on creating flexible manufacturing capabilities to support highly specialized, sometimes smaller-batch, therapies that require rapid turnaround times for patient-specific treatment. For instance, in February 2024, WuXi Advanced Therapies established a rapid-turnaround cell therapy manufacturing service in Philadelphia, supporting patient-specific autologous therapies with an average vein-to-vein delivery of just 9 days for rare leukemia indications. This focus on speed and customization is crucial for improving patient outcomes and ensuring the sustainable growth of this specialized market segment.
The North America Cell and Gene Therapy CDMO Market is positioned for a remarkable period of expansion, driven by scientific breakthroughs, an encouraging regulatory environment, and massive financial investment. The foundational figures, as detailed in the Credence Research data a projected leap from $2.8 billion in 2024 to over $11.1 billion by 2032 confirm the sector’s status as a top growth area in North America Biotech.
By addressing the complex challenges of scalability, embracing digital manufacturing, and fostering deep-seated strategic collaborations, CDMOs are not just service providers but essential innovation partners. As the pipeline of advanced therapies continues to move through clinical trials toward commercialization, the specialized capabilities and capacity of the CDMO ecosystem will remain the indispensable engine driving the next generation of curative medicine into the hands of patients. The future of personalized and genetic medicine is inextricably linked to the continued success and evolution of the North America Cell and Gene Therapy CDMO Market.
Source: https://www.credenceresearch.com/report/north-america-cell-and-gene-therapy-cdmo-market
- Business
- Research
- Energy
- Art
- Causes
- Tech
- Crafts
- crypto
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness