Intraosseous Infusion Devices Market Revenue, Growth, Developments, Size, Share and Forecast 2025 To 2032
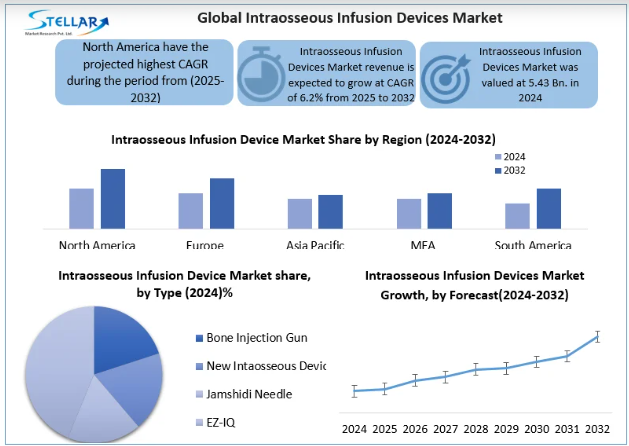
Intraosseous Infusion Devices Market Size was valued at USD 5.43 Bn. In 2024, the total Intraosseous Infusion Devices revenue is expected to grow by CAGR 6.2% from 2025 to 2032 and reach nearly USD 8.79 Bn. In 2032.
Market Estimation & Definition
Intraosseous infusion devices are specialized medical tools designed to deliver fluids, medications, and blood products directly into the bone marrow cavity when conventional intravenous access fails — especially during critical emergency scenarios such as cardiac arrest, severe trauma, or shock. This direct infusion route bypasses collapsed or difficult veins and ensures rapid systemic delivery.
These devices range from needles and impact‑driven tools to automated battery‑powered insertion systems. They are used across emergency departments, ambulance services, military medical units, trauma centers, and surgical settings. Their life‑saving capabilities have led to increasing clinical adoption throughout Europe.
Healthcare professionals value IO devices for their ability to reduce procedure time, enhance clinical efficiency, and support high‑pressure emergency interventions.
Obtain your sample copy of this report now! https://www.stellarmr.com/report/req_sample/intraosseous-infusion-devices-market/2705
Market Growth Drivers & Opportunity
Key Market Growth Drivers
Increasing Emergency Response Demand
Rising incidence of trauma cases, out‑of‑hospital cardiac arrests, and other critical care scenarios has created strong demand for devices that provide rapid vascular access. Emergency responders, paramedics, and hospital teams are integrating intraosseous devices into standard practice because they offer a lifeline when IV access is delayed.
Government and Clinical Guidelines Encouragement
Across Europe, professional bodies and clinical guideline committees have increasingly recognized intraosseous access as a viable alternative to intravenous access when rapid access is essential. This clinical endorsement has encouraged adoption in both pre‑hospital and in‑hospital care settings.
Healthcare Infrastructure Investments
Ongoing investments in emergency medical services, trauma care readiness, and modern pre‑hospital care systems have supported increased procurement of intraosseous devices by hospitals, ambulance services, and emergency response units.
Technology Advancements
Continuous innovation in device design — such as ergonomic battery‑powered drivers and rapid‑insertion systems — enhances convenience, accuracy, and clinician confidence, further propelling market growth.
Market Opportunities
Expansion of EMS and Pre‑Hospital Care Systems
Upgrading ambulance fleets, mobile intensive care units, and emergency response networks presents significant opportunity for intraosseous device deployment, especially in regions prioritizing rapid and efficient critical care.
Training and Simulation Programs
As medical training evolves to include more advanced emergency protocols, IO device education and simulation tools are gaining traction, fostering higher adoption rates among first responders and medical teams.
Pediatric and Specialized Care Adoption
Specialized intraosseous systems tailored for pediatric and neonatal emergency cases are expanding the market reach, reflecting an unmet need in critical care scenarios involving children.
Public‑Private Collaborative Initiatives
Collaborations between manufacturers, healthcare institutions, and government agencies focused on trauma response preparedness are driving broader device accessibility and clinical utilization.
Segmentation Analysis
The intraosseous infusion devices market is segmented by product type, root of administration, and end user to reflect varied clinical needs and operational environments.
By Device Type
-
Manual Needles
-
Impact‑Driven Devices
-
Battery‑Powered Drivers
Each type offers specific advantages: manual needles are cost‑effective and simple, impact‑driven devices offer rapid insertion without power needs, and battery‑powered drivers provide automated, consistent access.
By Route of Administration
-
Sternum
-
Tibia (Proximal)
-
Humerus
-
Femur
The sternum is commonly used due to its rapid access and strategic anatomical location in emergency care scenarios, while other sites like the tibia and humerus are selected based on patient condition and procedural requirements.
By End User
-
Hospitals
-
Emergency Medical Services (EMS)
-
Military & Defense
-
Ambulatory Surgical Centers
-
Training & Education Centers
Hospitals and EMS typically represent the largest end‑use segments due to the frequency and urgency of clinical interventions where intraosseous access is needed.
To delve deeper into this research, kindly explore the following link: https://www.stellarmr.com/report/intraosseous-infusion-devices-market/2705
Country‑Level Analysis: Europe Focus
Germany
Germany stands as a leading market in Europe, supported by advanced trauma care infrastructure, high adoption of emergency medical technologies, and extensive paramedic training programs. The integration of IO access into clinical protocols has strengthened emergency response capabilities.
United Kingdom
The United Kingdom maintains a significant share of the regional market due to structured public healthcare investment, emphasis on advanced lifesaving protocols, and national guidelines that encourage intraosseous device use in pre‑hospital and hospital settings.
France
France’s well‑developed emergency care system and increasing focus on rapid, reliable vascular access contribute to strong IO device demand in both urban and regional healthcare facilities.
Italy and Spain
Italy’s aging demographic profile and Spain’s expanding emergency medical services support steady adoption of intraosseous devices, particularly within critical and ambulance care units.
Nordic & Rest of Europe
Countries such as Sweden and Austria are experiencing gradual uptake, driven by national healthcare improvements and targeted emergency response training initiatives.
Commutator Analysis
In the intraosseous infusion devices market, key stakeholders shaping demand and usage include:
Emergency Medical Services
Pre‑hospital care providers rely heavily on IO devices to secure rapid access in life‑threatening situations, especially when intravenous access is difficult.
Hospitals and Trauma Centers
Hospitals leverage intraosseous systems in emergency departments, intensive care units, and surgical settings as a reliable fallback for vascular access.
Military and Defense Medical Units
Field medical teams and defense healthcare units deploy IO devices as essential tools in battlefield trauma care and disaster response preparations.
Training & Education Institutions
Medical training centers and professional certification programs that include IO access skills contribute to broader clinical acceptance and proper usage standards.
Unlock Free Market Inquiry
North America Exosome Research Market https://www.stellarmr.com/report/North-America-Exosome-Research-Market/784
Europe Exosome Research Market https://www.stellarmr.com/report/Europe-Exosome-Research-Market/826
Press Release Conclusion
The Europe Intraosseous Infusion Devices Market is positioned for meaningful expansion through 2032 as emergency care protocols evolve and healthcare systems emphasize rapid, reliable vascular access solutions. Rising case volumes in trauma, cardiac emergencies, and critical care events, combined with technology enhancements, training adoption, and supportive clinical guidelines, are driving sustained demand across hospitals, EMS units, and specialized medical centers.
With opportunities emerging in pre‑hospital modernization, training infrastructure, and pediatric care sectors, the market offers compelling potential for healthcare stakeholders and technology innovators focused on improving emergency medical outcomes.
About Stellar Market Research
Stellar Market Research is a global leader in market research and consulting, providing data‑driven insights, strategic analysis, and competitive evaluation across industries including healthcare, medical devices, technology, and consumer goods. The firm empowers organizations with actionable intelligence to navigate dynamic markets and achieve growth.
For More Information, Please Contact:
Stellar Market Research
S.no.8, H.no. 4‑8 Pl.7/4, Kothrud
Pinnac Memories Fl. No. 3, Kothrud
Pune, Maharashtra, 411029, India
📧 sales@stellarmr.com
📞 +91 20 6630 3320 | +91 9607365656
- Intraosseous_Infusion_Devices_Market
- Intraosseous_Infusion_Devices_Market_Trends
- Intraosseous_Infusion_Devices_Market_Analysis
- Intraosseous_Infusion_Devices_Market_Report
- Intraosseous_Infusion_Devices_Market_Overview
- Intraosseous_Infusion_Devices_Market_Revenue
- Intraosseous_Infusion_Devices_Market_Opportunities
- Business
- Research
- Energy
- Art
- Causes
- Tech
- Crafts
- crypto
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


